Abstract
Introduction: Allogeneic hematopoietic cell transplantation (HCT) is a standard therapy for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS); the outcomes of patients who relapse following HCT are dismal. HCT can suppress the progression of AML or MDS through a graft-vs-leukemia (GvL) effect, and in this manner, cure a subset of patients. In post-HCT relapse, agents with direct anti-leukemic activity, but which also enhance GvL, are of interest. High dose lenalidomide (50mg daily) had a promising response rate in relapsed AML (Blum et al JCO 2010), and has immunomodulatory properties that may be particularly effective in the post-HCT setting. Bortezomib has been shown to potentiate other chemotherapies and may augment GvL while limiting graft vs. host disease (GVHD). We studied increasing doses of bortezomib added to lenalidomide for patients with AML or MDS relapsing after HCT.
Methods: Patients aged 18 or older with AML or MDS relapsing after HCT were enrolled on this phase I '3+3' dose-escalation study of increasing doses of bortezomib combined with lenalidomide. Patients had to be off immunosuppressive therapy for 2 weeks; <20mg/day prednisone was allowed. Patients could not have grade (G) ≥3 acute GVHD or chronic GVHD requiring > 20mg/day prednisone. Patients received lenalidomide at a dose of 50mg, on days (d) 1-21 of 28-day induction cycles. On days 2, 5, 9, and 12, they received subcutaneous bortezomib exploring 3 dose levels: 0.7mg/m2, 1.0mg/m2, and 1.3mg/m2. Patients could receive up to two cycles of induction.
Dose limiting toxicities (DLTs) included G4 peripheral neuropathy, G3 or higher aGVHD, moderate/severe cGVHD, or other G3/4 non-hematologic toxicities that did not resolve to G≤2 by the end of the cycle. Inability to receive at least 50% of both drugs during induction was a DLT. Hematologic toxicities with active AML/MDS were not DLTs. The DLT period spanned up to 2 induction cycles.
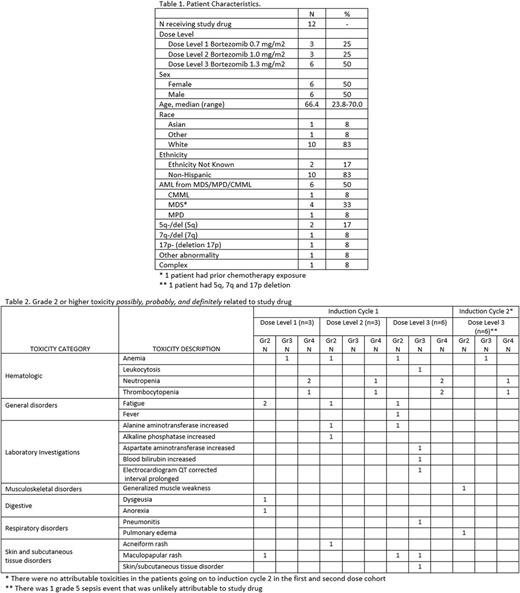
Results: 13 patients were enrolled during the dose escalation portion of the study. One patient died from progressive AML before therapy and was replaced for DLT evaluation and analysis. The median age at registration was 66 years (range 23-70); 50% were male, and all 12 eligible patients had an underlying diagnosis of AML, 6 being secondary AML (Table 1). At baseline, none of the 10 tested had a FLT3-ITD mutation or an NPM1 mutation. At transplant, patients were in CR1 (n=6), CR2 (n=1), refractory disease (n=3); 2 did not have reported disease status. The median time from HCT to study enrollment was 0.9 years (0.3-6.5 years).
All 12 patients received lenalidomide 50mg daily in the first induction cycle, along with the assigned bortezomib dose; 11 patients had the lenalidomide and/or bortezomib doses held due to toxicity during induction cycle 1. A total of 9 patients (2 in cohort 1, 1 in cohort 2, 6 in cohort 3) continued onto induction cycle 2; 4 (44%) received a reduced dose of lenalidomide at 25mg d1-21. There were no DLTs at dose levels 1 or 2; at dose level 3, patient 7 experienced a DLT for receiving < 50% of doses. Dose level 3 was thus expanded; no further DLTs were seen. However, there were several G3/4 attributable toxicities (Table 2) not meeting DLT criteria, including neutropenia (n=6), thrombocytopenia (n=5), pleural effusion (n=1), rash (n=2), muscle weakness (n=1), AST elevation (n=1), bilirubin (n=1) elevation, and QT prolongation (n=1). One patient developed aGVHD during induction 1 (stage 1 skin + liver, overall grade II); this patient achieved a CRi. Two patients developed cGVHD during cycle 2 of induction (liver in both; one with lung, joint, and fascia). One patient in cohort 3 died of sepsis during induction cycle 2, not attributed to study drug. Most patients had treatment held due to toxicity. The total number of bortezomib doses given during induction cycle 1 was 2/4 doses n=2; 3/4 n=5; 4/4 n=5; and cycle 2 was 2/4 n=1; 3/4 n=3; 4/4 n=5; the median doses of lenalidomide received during induction cycle 1 was 15 (range 7-21) and during induction cycle 2 was 18 (3-21).
Conclusions: The recommended phase II dose of bortezomib combined with lenalidomide 50mg d1-21/28 for patients relapsing with AML or MDS post-HCT is 1.3mg/m2 on days 2, 5, 9, and 12. Few toxicities attributable to these drugs were noted during induction therapy; however, toxicity in this post-HCT relapse setting is high and a number of patients required dose modifications. The efficacy of this regimen is currently under evaluation.
Brunner: Celgene: Research Funding; Takeda: Research Funding. Avigan: Genus Oncology: Research Funding. Fathi: Pfizer: Honoraria; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; Medimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal